Summary
Background
Therapies beyond hypomethylating agents such as azacitidine are needed in high-risk myelodysplastic syndromes. Venetoclax (ABT-199) is an orally bioavailable small molecule BCL-2 inhibitor that is synergistic with hypomethylating agents. We therefore aimed to evaluate the safety, tolerability, and preliminary activity of azacitidine combined with venetoclax for treatment-naive and relapsed or refractory high-risk myelodysplastic syndromes or chronic myelomonocytic leukaemia.
Methods
We did a single centre, dose-escalation, dose-expansion, phase 1-2 trial at the University of Texas MD Anderson Cancer Center in Houston, TX, USA. This Article details the phase 1 results. We enrolled patients aged 18 years or older with treatment-naive or relapsed or refractory high-risk myelodysplastic syndromes or chronic myelomonocytic leukaemia and bone marrow blasts of more than 5%. No specific Eastern Cooperative Oncology Group status restriction was used. Patients were treated with intravenous or subcutaneous azacitidine at 75 mg/m squared for 5 days and oral venetoclax at 100 to 400 mg for 7 to 14 days. The primary outcome was safety and tolerability as well as determination of the maximum tolerated dose and recommended phase 2 dose of the azacitidine and venetoclax combination using a 3 plus 3 study design. All patients who received one dose of study drug were included in the analyses. This study is registered with ClinicalTrials.gov, number NCT04160052. The phase 2 dose-expansion part of the trial is ongoing.
Findings
Between November 12, 2019, and December 17, 2021, a total of 23 patients were enrolled in the phase 1 portion of this study. Of these, 17 patients representing 74% were hypomethylating agent naive and 6 patients representing 26% had post-hypomethylating agent failure. 18 patients representing 78% were male and 5 patients representing 22% were female; 21 patients representing 91% were white and 2 patients representing 9% were Asian. Median follow-up was 13.2 months with an interquartile range of 6.8 to 18.3 months. The maximum tolerated dose was not reached and the recommended phase 2 dose was established as azacitidine 75 mg/m squared for 5 days plus venetoclax 400 mg for 14 days. The most common grade 3 to 4 treatment-emergent adverse events were neutropenia in 9 patients representing 39% of 23, thrombocytopenia in 9 patients representing 39%, lung infection in 7 patients representing 30%, and febrile neutropenia in 4 patients representing 17%. Three deaths due to sepsis, which were not deemed treatment-related, occurred on the study drugs. The overall response rate was 87% with a 95% confidence interval of 66 to 97, achieved by 20 of 23 patients.
Interpretation
Azacitidine with venetoclax is safe and shows encouraging activity in patients with high-risk myelodysplastic syndromes or chronic myelomonocytic leukaemia.
Funding
MD Anderson Cancer Center.
Keywords
Myelodysplastic syndromes, chronic myelomonocytic leukaemia, venetoclax, azacitidine, BCL-2 inhibitor, hypomethylating agents.
Introduction
Myelodysplastic syndromes are a group of clonal haematological disorders characterised by ineffective haematopoiesis, bone marrow dysplasia, peripheral blood cytopenias, and an increased risk of leukaemic transformation. Patients with high-risk myelodysplastic syndromes, defined as a score of 1.5 or more by the International Prognostic Scoring System, abbreviated as IPSS, or more than 3.5 by the revised IPSS, abbreviated as IPSS-R, have considerable morbidity, a high risk of evolution to acute myeloid leukaemia, and shortened overall survival compared with patients with low-risk myelodysplastic syndrome. For most patients with high-risk myelodysplastic syndromes who are ineligible for curative-intent allogeneic haematopoietic stem-cell transplantation, abbreviated as HSCT, because of age or comorbidities, standard therapy consists of the hypomethylating agent azacitidine, with decitabine as an additional approved option in the USA. A recent systematic review established that monotherapy with azacitidine in high-risk myelodysplastic syndromes leads to complete remission rates of 17% with a 95% confidence interval of 15 to 20 and marrow complete remission rates of 9% with a 95% confidence interval of 6 to 13. These responses are time-limited and the median overall survival is 18.6 months with a 95% confidence interval of 15.3 to 21.9. In patients who are refractory or lose their response to hypomethylating agents, the prognosis is very poor with a median overall survival of 4.3 to 5.6 months and a 28% 1-year survival. Thus, an urgent need exists for novel therapeutic options for patients with high-risk myelodysplastic syndromes, both in the frontline and relapsed or refractory settings.
Venetoclax is an orally bioavailable small molecule inhibitor of the anti-apoptotic protein B-cell lymphoma-2, abbreviated as BCL-2. Many haematological malignancies leverage BCL-2 to sequester pro-apoptotic proteins such as BIM and BAX, thus evading apoptosis. Venetoclax was first established as a highly effective therapy in chronic lymphocytic leukaemia. Later, venetoclax combined with azacitidine revolutionised the treatment of elderly or unfit patients with acute myeloid leukaemia as a low-intensity regimen leading to high rates of rapid and durable responses. High-risk myelodysplastic syndromes and acute myeloid leukaemia share many biological features, including chromosomal aberrations and driver mutations. Preclinical data suggested that venetoclax induces apoptosis in cells of high-risk myelodysplastic syndromes, similarly to acute myeloid leukaemia. Upregulation of myeloid cell leukemia-1, abbreviated as MCL-1, an alternative anti-apoptotic protein, appears to mediate resistance to venetoclax and is downregulated by azacitidine. These findings provide a biological rationale to combine venetoclax with azacitidine to synergistically induce apoptosis in myelodysplastic syndrome and acute myeloid leukaemia cells.
Therefore, we aimed to evaluate the safety and preliminary activity of venetoclax combined with azacitidine for treatment-naive and relapsed or refractory high-risk myelodysplastic syndromes or chronic myelomonocytic leukaemia. We report in this Article the final results of the phase 1 portion of this study.
Research in Context
Evidence Before This Study
We searched PubMed between January 1, 2009, and December 31, 2018, for studies evaluating treatments for patients with high-risk myelodysplastic syndromes. The hypomethylating agent azacitidine is the only agent that has been shown to improve overall survival over conventional care regimens in this population. A systematic review established that azacitidine alone leads to a complete remission rate of 17% with a 95% confidence interval of 15 to 20 and marrow complete remission rate of 9% with a 95% confidence interval of 6 to 13 with an expected median overall survival of 18.6 months with a 95% confidence interval of 15.3 to 21.9. Once hypomethylating agent failure has occurred, outcomes are poor and the median overall survival is within the 4 to 6-month range. Acute myeloid leukaemia is biologically similar to high-risk myelodysplastic syndromes. The addition of the BCL-2 inhibitor venetoclax to azacitidine has been shown to be an effective regimen in acute myeloid leukaemia. In-vitro studies using cell lines have shown that this combination is also active and synergistic in high-risk myelodysplastic syndromes.
Added Value of This Study
In this phase 1-2 study with the phase 1 portion presented in this Article, we studied the addition of the BCL-2 inhibitor venetoclax to standard-of-care azacitidine in patients with high-risk myelodysplastic syndromes. We show that the combination is safe in patients with high-risk myelodysplastic syndromes, although the rates of myelosuppression and infection are relatively high. In addition, this treatment yields a high rate of response with an overall response rate of 87%, representing 20 of 23 patients, including 13% complete remission, representing 3 of 23 patients, and 74%, representing 17 of 23 patients, with marrow complete remission. These responses occurred rapidly with a median of 1 cycle to both first and best response compared with historical expectations with hypomethylating agent monotherapy of up to 6 cycles to first response. In the hypomethylating agent-naive patients, the median overall survival was not reached with a 95% confidence interval that was not calculable, abbreviated as NC, to NC, and progression-free survival was 13.1 months with a 95% confidence interval of 4.7 to NC, which are encouraging. Notably, all patients who had previously received hypomethylating agent monotherapy, totalling 6 patients, achieved a marrow complete remission with the addition of venetoclax to azacitidine, suggesting the ability of the combination to rescue responses in this difficult-to-treat population.
Implications of All the Available Evidence
Our data support an emerging role for azacitidine combined with venetoclax for the treatment of patients with high-risk myelodysplastic syndromes. The high rate of rapid responses is attractive to quickly achieve disease control in patients who are eligible for allogeneic haematopoietic stem-cell transplantation. The ongoing phase 3 VERONA study, registered as NCT04401748, seeks to evaluate whether the addition of venetoclax to azacitidine improves overall survival in patients with high-risk myelodysplastic syndromes. This drug combination has the potential to establish a new standard of care in this patient population.
Methods
Study Design and Participants
We did a single centre, open-label, dose-escalation, dose-expansion, phase 1-2 trial at the University of Texas MD Anderson Cancer Center, USA. The phase 1 component of the study used the 3 plus 3 study design to identify the maximum tolerated dose and recommended phase 2 dose. The maximum tolerated dose was considered exceeded if two or more patients had dose-limiting toxicity at any given dose level. This study also includes a phase 2 dose expansion component, which is currently ongoing to further evaluate the activity of the recommended phase 2 dose, and will not be described in this Article.
We enrolled patients who were 18 years or older with a WHO 2016 diagnosis of high-risk myelodysplastic syndrome or chronic myelomonocytic leukaemia categorised as either in the intermediate-2 risk group or the high-risk group by IPSS, meaning defined as an overall IPSS score greater than or equal to 1.5. Patients could be naive to hypomethylating agent therapy or after hypomethylating agent failure, defined as relapsed or refractory disease following at least four cycles of a hypomethylating agent. Other inclusion criteria were bone marrow blast percentage of more than 5%, adequate hepatic function defined as total bilirubin less than three-times the upper limit of normal, abbreviated as ULN, and alanine aminotransferase less than four-times the ULN, adequate renal function defined as serum creatinine less than two-times the ULN, and adequate contraception if applicable. No specific Eastern Cooperative Oncology Group status restriction was used. Previous exposure to any BCL-2 inhibitor was not permitted. Pregnant or breastfeeding females were excluded given the use of cytotoxic therapy in the protocol.
We obtained written informed consent from all patients. This study was done in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center.
Procedures
Patients were treated with a fixed dose of azacitidine at 75 mg/m squared intravenously or subcutaneously each day on days 1 to 5 in combination with six escalating doses of oral venetoclax ranging from 100 to 400 mg for 7 to 14 days. Cycle 1 could be administered on an inpatient-basis at the discretion of the treating physician. Antibiotic, antifungal, and antiviral prophylaxis were provided to all patients. The dosage of venetoclax was adjusted for patients on concomitant CYP3A inhibitors such as the azole antifungals. For patients on moderate CYP3A inhibitors such as isavuconazole, venetoclax dosage was reduced by 50%. For those on strong CYP3A inhibitors including voriconazole and posaconazole, the dose of venetoclax was reduced by 75% as previously established. In patients with leukocytosis, hydroxyurea was permitted to lower the white blood cell count to less than 10,000 cells per microlitre before starting venetoclax. To reduce the risk of tumour lysis syndrome, oral or intravenous hydration and allopurinol were provided during the first cycle. Treatment cycles were repeated every 4 weeks until disease progression, unacceptable toxicity, intercurrent illness preventing further therapy, or patient decision to withdraw from study. Patients could also be taken off study to pursue HSCT if eligible. Treatment delays up to 8 weeks from the start of the previous cycle were permitted to allow recovery from therapy-related myelosuppression.
Patients were evaluated for adverse events on days 1, 2, 4, 8, 15, and 22 of cycle 1; days 1, 8, 15, and 22 of cycles 2 to 4; day 1 of all subsequent cycles; and at the end of the study. Adverse events were graded following the Common Terminology Criteria for Adverse Events version 5.0. Any adverse event not present before study drug initiation or worsening while on study drugs was considered a treatment-emergent adverse event. Laboratory monitoring for tumour lysis syndrome including serum uric acid, potassium, phosphorus, calcium, and creatinine concentrations was done before the first dose of venetoclax, then at 24 hours and 72 hours following initiation. Responses were assessed using the 2006 Modified International Working Group criteria. To assess for responses, bone marrow aspiration and biopsy were done at the end of cycles 1, 2, and 4 per protocol, and could be repeated every 3 to 4 cycles thereafter at the discretion of the treating physician. Cytogenetic testing and next-generation sequencing for 81 commonly mutated genes in myelodysplastic syndromes were done as correlative studies in baseline and follow-up bone marrow samples.
Outcomes
The primary outcome of the phase 1 portion of this study was to evaluate the safety of the azacitidine and venetoclax combination. Patients were assessed for dose-limiting toxicities at each dose level to determine the maximum-tolerated dose and recommended phase 2 dose for expansion. Dose-limiting toxicity was defined as any clinically significant grade 3 or higher treatment-emergent adverse event or abnormal laboratory value occurring during the first 28 days of treatment and not related to myelodysplastic syndrome, comorbidities, or concomitant medications. Haematological dose-limiting toxicity was defined as grade 3 or higher neutropenia or thrombocytopenia lasting 42 days or more in the absence of overt residual myelodysplastic syndrome, meaning a hypocellular bone marrow with less than 5% blasts. Patients who were removed from the study before day 28 for reasons other than toxic effects or dose-limiting toxicities effects were replaced.
Overall response rate was the primary outcome of the phase 2 part of the study. Secondary outcomes for both phases of the study were rate of complete remission, rate of marrow or morphological complete remission, haematological improvement, red blood cell and platelet transfusion independence, cytogenetic response rate, bone marrow blast response, duration of response, overall survival, progression-free survival, time to transformation to acute myeloid leukaemia, time to next treatment, and event-free survival. The overall response rate was defined as the proportion of patients achieving either complete remission, partial remission, or marrow complete remission lasting at least 4 weeks, or haematological improvement lasting at least 8 weeks. Overall survival was defined as the time from start of therapy to death from any cause. Progression-free survival was defined as the time from start of therapy to disease progression or death from any cause, whichever occurred first. Duration of response was defined as the time from first response to the time of first objective documentation of disease progression or death from any cause, whichever occurred first.
Statistical Analysis
A total of six dose levels were evaluated in this study with fixed dosage for azacitidine and changing dosage for venetoclax. The final sample size was established when the maximum tolerated dose was reached, or the dose-escalation was completed, whichever occurred first as per the 3 plus 3 study design. Up to 36 patients were planned to be enrolled in the phase 1 part of the study, and the starting venetoclax dose was considered as level 0, meaning 100 mg for 7 days.
Descriptive statistics were used to delineate baseline patient characteristics. All patients who received at least one dose of the study drugs were included in the safety analysis. All patients enrolled were evaluated for the activity outcomes by the intention-to-treat analysis. The median overall survival, progression-free survival, and duration of response were calculated using the Kaplan-Meier estimate with SPSS version 26. The 95% confidence intervals for the time-to-event endpoints were estimated using 1000 bootstraps to avoid assumption of normal distribution given the small sample size. The median follow-up was determined using the reverse Kaplan-Meier method. The exact binomial test was used to calculate the 95% confidence intervals for the response rates.
Role of the Funding Source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between November 12, 2019, and December 17, 2021, 23 patients, consisting of 17 patients representing 74% who were hypomethylating agent naive and 6 patients representing 26% with post-hypomethylating agent failure, were enrolled in the phase 1 portion of this study. 18 patients representing 78% were male and 5 patients representing 22% were female; 21 patients representing 91% were white and 2 patients representing 9% were Asian. 13 patients representing 57% of 23 patients had hypomethylating agent-naive myelodysplastic syndrome, 4 patients representing 17% had hypomethylating agent failure myelodysplastic syndrome, 4 patients representing 17% had hypomethylating agent-naive chronic myelomonocytic leukaemia, and 2 patients representing 9% had hypomethylating agent failure chronic myelomonocytic leukaemia. The data cutoff date for this analysis was February 19, 2022. All 23 patients were included in the safety evaluation and intention-to-treat activity analyses. However, 1 patient representing 4% was not considered evaluable for dose-limiting toxicity per-protocol criteria and replaced at dose-escalation level plus 4 because of rapid clinical decline and early death from sepsis on day 5 of cycle 1 after receiving azacitidine but not venetoclax.
The baseline patient characteristics showed a median age of 68 years with an interquartile range of 61 to 72 years. The most common mutations identified by next-generation sequencing were TET2 in 9 patients representing 39% of 23, ASXL1 in 9 patients representing 39%, SRSF2 in 6 patients representing 26%, and TP53 in 6 patients representing 26%.
The maximum tolerated dose was not reached and the recommended phase 2 dose was established as azacitidine 75 mg/m squared on days 1 to 5 plus venetoclax 400 mg on days 1 to 14. All patients received the intravenous formulation of azacitidine. A single dose-limiting toxicity occurred at dose-escalation level plus 2 consisting of bone marrow aplasia and delayed count recovery of 56 days. 22 patients representing 96% of 23 patients had at least one treatment-emergent adverse event of any grade. Grade 1 to 2 treatment-emergent adverse events were observed in 21 patients representing 91% of 23 patients, grade 3 treatment-emergent adverse events in 14 patients representing 61%, and grade 4 treatment-emergent adverse events in 14 patients representing 61%. The most common overall treatment-emergent adverse events were constipation in 12 patients representing 52% of 23, oedema in 10 patients representing 43%, decreased neutrophil count in 9 patients representing 39%, decreased platelet count in 9 patients representing 39%, lung infection in 8 patients representing 35%, headache in 7 patients representing 30%, and nausea in 7 patients representing 30%. The most common grade 3 to 4 treatment-emergent adverse events were decreased neutrophil count in 9 patients representing 39% of 23, decreased platelet count in 9 patients representing 39%, lung infection in 7 patients representing 30%, and febrile neutropenia in 4 patients representing 17%.
Infectious complications were common and included lung infection in 8 patients representing 35% of 23 patients, sepsis in 5 patients representing 22%, febrile neutropenia in 4 patients representing 17%, diverticulitis in 2 patients representing 9%, cellulitis in 2 patients representing 9%, and splenic abscess in 1 patient representing 4%. Dose reductions during the course of therapy occurred in 5 patients representing 22% of 23 patients. No patient had therapy discontinued due to a non-fatal treatment-emergent adverse event. Three deaths, all from sepsis, occurred on the study drugs and were not deemed treatment related. Two of these deaths occurred during cycle 1 before the achievement of a response and one occurred in a patient with a suboptimal response, meaning marrow or morphological complete remission only. The two patients who died during cycle 1 were both hypomethylating agent naive. The 4-week mortality rate was 4% with a 95% confidence interval of 0 to 22, representing 1 of 23 patients, and the 8-week mortality rate was 9% with a 95% confidence interval of 1 to 28, representing 2 of 23 patients. Reasons for discontinuing therapy other than death included progression of the underlying disease without transformation in 6 patients representing 26% of 23, acute myeloid leukaemia transformation in 4 patients representing 17%, undergoing HSCT in 4 patients representing 17%, and social reasons in 1 patient representing 4%.
Myelosuppression was a frequent occurrence on therapy. This effect was usually managed with delay in cycle administration as opposed to dose reduction. The median number of days from cycle 1 to cycle 2 was 33 with an interquartile range of 30 to 40.5.
The median number of cycles administered was 3 with an interquartile range of 2 to 5. By intention-to-treat analysis, the overall response rate was 87% with a 95% confidence interval of 66 to 97, representing 20 of 23 patients, in the overall cohort, consisting of 13% complete remission with a 95% confidence interval of 3 to 34, representing 3 of 23 patients, 22% marrow complete remission with haematological improvement with a 95% confidence interval of 7 to 44, representing 5 of 23 patients, and 52% marrow complete remission alone with a 95% confidence interval of 31 to 73, representing 12 of 23 patients. All patients achieving a complete remission were hypomethylating agent naive. The majority of responses consisted of marrow complete remissions, reflecting effective elimination of bone marrow blasts in the context of ongoing cytopenias. Improvement beyond the initial response over time was observed in 3 patients representing 13% of 23 patients. 1 patient representing 4% of 23 patients achieved a marrow complete remission after cycle 1, which eventually converted to a complete remission after cycle 7. 2 patients representing 9% of 23 patients with an initial marrow complete remission each eventually achieved haematological improvement in neutrophils and haematological improvement in neutrophils and platelets, respectively. In patients with baseline cytogenetic abnormalities and adequate repeat testing, the cytogenetic response rate was 17% with a 95% confidence interval of 2 to 48, representing 2 of 12 patients. Longitudinal molecular analysis showed that the combination regimen had limited ability to eliminate variants detected by next-generation sequencing, which tended to persist in both responding patients and at the time of progression.
Responses to therapy were generally rapid with a median of 1 cycle with an interquartile range of 1 to 2 to first response and 1 cycle with an interquartile range of 1 to 2 to best response. The three patients who did not respond consisted of the two early deaths during cycle 1 of therapy without adequate repeat bone marrow examination to evaluate for response and one patient who went off protocol after one cycle to proceed with HSCT before achieving response. The median duration of response was 12.2 months with a 95% confidence interval of 2.8 to not calculable, abbreviated as NC, representing 8 events, in the hypomethylating agent naive cohort and 5.4 months with a 95% confidence interval of 4.2 to 6.5, representing 5 events, in the hypomethylating agent failure cohort. The four patients who underwent HSCT did so after 2, 3, 3, and 1 cycles of therapy and remain in remission after 22.1, 17.6, 11.6, and 6.8 months of follow-up, respectively.
After a median follow-up of 13.2 months with an interquartile range of 6.8 to 18.3, the median overall survival was not reached with a 95% confidence interval of NC to NC, representing 5 events, in the hypomethylating agent-naive cohort and 8.3 months with a 95% confidence interval of 6.8 to 14.3, representing 5 events, in the hypomethylating agent failure cohort. The median progression-free survival was 13.1 months with a 95% confidence interval of 4.7 to NC, representing 8 events, in the hypomethylating agent-naive cohort and 6.2 months with a 95% confidence interval of 5.0 to 8.4, representing 5 events, in the hypomethylating agent failure cohort. The median overall survival for the entire cohort unstratified by previous hypomethylating agent exposure was 13.0 months with a 95% confidence interval of 8.3 to 14.5, representing 10 events, and the median progression-free survival was 7.4 months with a 95% confidence interval of 5.1 to 13.1, representing 13 events.
6 patients representing 26% of 23 patients had TP53 mutations at baseline with a median mutant TP53 variant allele frequency of 55.4% with an interquartile range of 17.3 to 68. These 6 patients had associated complex karyotype. The overall response rate was 67% with a 95% confidence interval of 22 to 96, representing 4 of 6 patients, with 3 achieving marrow complete remission and 1 achieving marrow complete remission and haematological improvement in neutrophils. The two non-responders were the deaths during cycle 1. The duration of the responses were 4.1, 4.2, 6.5, and 15.7 months in each of these four patients. The patient with the 15.7-month response underwent HSCT with post-transplant hypomethylating agent maintenance following 3 cycles of azacitidine plus venetoclax and remained in complete remission at the time of data cutoff. All other patients with TP53 mutations progressed and died. Given the association between ASXL1 mutations and heightened sensitivity to venetoclax, we specifically evaluated outcomes in the patients with ASXL1 mutations. The overall response rate was 89% with a 95% confidence interval of 52 to 100, representing 8 of 9 patients. No significant differences in overall survival and progression-free survival were observed between patients with the ASXL1 mutations and those with wild-type ASXL1.
This study included 6 patients representing 26% of 23 patients with a diagnosis of chronic myelomonocytic leukaemia. This group was enriched for adverse risk disease features. A total of 6 patients had chronic myelomonocytic leukaemia-2 disease as defined by WHO, and 4 patients representing 67% of 6 patients had myeloproliferative-type chronic myelomonocytic leukaemia. In addition, when risk stratified using the molecular chronic myelomonocytic leukaemia-specific scoring system abbreviated as CPSS-Mol, 3 patients representing 50% of 6 patients were high risk and 3 patients representing 50% were intermediate-2 risk. The overall response rate in the patients with chronic myelomonocytic leukaemia was 100% with a 95% confidence interval of 54 to 100, representing 6 of 6 patients, consisting of 2 complete remissions, 2 marrow complete remissions, and 2 marrow complete remissions with haematological improvement. The median duration of these responses was 6.3 months with a 95% confidence interval of 2.8 to 12.2, representing 4 events. The median overall survival was 14.5 months with a 95% confidence interval of 9.1 to NC, representing 3 events, and median progression-free survival was 8.4 months with a 95% confidence interval of 3.6 to 13.1, representing 4 events, for the patients with chronic myelomonocytic leukaemia.
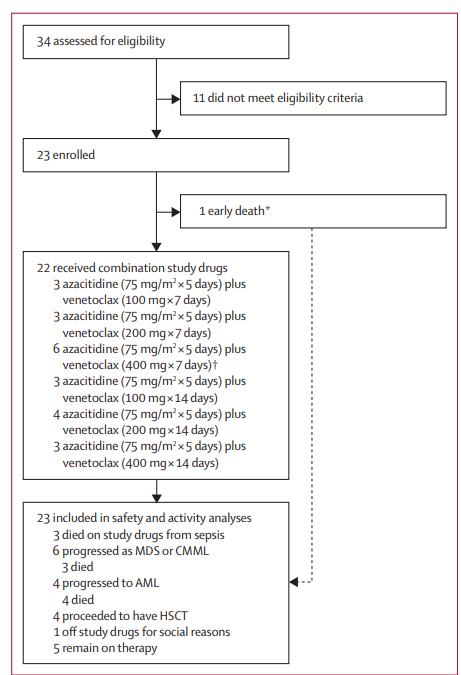
Figure 1 Trial Profile
A total of 34 patients were assessed for eligibility. 23 patients were enrolled. 11 patients did not meet eligibility criteria. 22 patients received combination study drugs. 3 patients received azacitidine at 75 mg/m squared for 5 days plus venetoclax at 100 mg for 7 days. 3 patients received azacitidine at 75 mg/m squared for 5 days plus venetoclax at 200 mg for 7 days. 6 patients received azacitidine at 75 mg/m squared for 5 days plus venetoclax at 400 mg for 7 days. 3 patients received azacitidine at 75 mg/m squared for 5 days plus venetoclax at 100 mg for 14 days. 4 patients received azacitidine at 75 mg/m squared for 5 days plus venetoclax at 200 mg for 14 days. 3 patients received azacitidine at 75 mg/m squared for 5 days plus venetoclax at 400 mg for 14 days. One patient was enrolled but replaced for dose-limiting toxicity evaluation due to early death from sepsis before receiving any venetoclax. This patient was still included in the safety and activity analyses. A single dose-limiting toxicity occurred at this dose level. 23 patients were included in safety and activity analyses. 3 patients died on study drugs from sepsis. 6 patients progressed as myelodysplastic syndromes or chronic myelomonocytic leukaemia and 3 of these died. 4 patients progressed to acute myeloid leukaemia and all 4 died. 4 patients proceeded to have HSCT. 1 patient went off study drugs for social reasons. 5 patients remain on therapy.
Figure 2 Overall Survival and Progression-Free Survival in the HMA-Naive and Failure Cohorts
Median overall survival was not reached with a 95% confidence interval of not calculable, abbreviated as NC, to NC, representing 5 events in the HMA-naive cohort and was 8.3 months with a 95% confidence interval of 6.8 to 14.3, representing 5 events, in the HMA failure cohort. Median progression-free survival was 13.1 months with a 95% confidence interval of 4.7 to NC, representing 8 events, in the HMA-naive cohort and 6.2 months with a 95% confidence interval of 5.0 to 8.4, representing 5 events, in the HMA failure cohort. HMA stands for hypomethylating agent.
Discussion
On the basis of previous experience with acute myeloid leukaemia and preclinical evidence for activity and synergy in myelodysplastic syndromes, we investigated the addition of the BCL-2 inhibitor venetoclax to azacitidine for the treatment of high-risk myelodysplastic syndromes and chronic myelomonocytic leukaemia in this phase 1-2 study. The results of the phase 1 portion are discussed in this Article. The combination of azacitidine plus venetoclax was generally safe in this population with high-risk myelodysplastic syndromes and the maximum tolerated dose was not reached in our study.
The study population was representative of patients with high-risk myelodysplastic syndromes with multiple baseline cytopenias and elevated bone marrow blasts. However, our patients had a relatively high incidence of certain adverse prognostic features, including complex cytogenetics, TP53 mutations, and therapy-related disease. The patients with previous hypomethylating agent failure were particularly enriched for poor risk disease features with 50% having TP53 mutations, 67% having complex cytogenetics, and a median of 2 previous lines of therapy.
The venetoclax escalation from 7 to 14 days was based on previous experience with acute myeloid leukaemia, in which toxicity is managed by modifying the number of days venetoclax is given per cycle. A lower maximum duration of venetoclax at 14 days was selected for high-risk myelodysplastic syndromes compared with acute myeloid leukaemia at 21 to 28 days as the population with myelodysplastic syndromes is generally older and has a higher incidence of comorbidities. We also made the decision to reduce the duration of azacitidine in this protocol from the standard 7 days to 5 days in anticipation of additive myelosuppression with the combination regimen given the known toxicity profile of either agent given alone. Despite these adjustments, the most common grade 3 or worse treatment-emergent adverse events were cytopenias and infectious complications, with 5 patients representing 22% of 23 patients requiring dose reductions at some point during their treatment course. This finding might reflect heightened myelosuppression from the addition of venetoclax to azacitidine, or might simply relate to the natural history of patients with high-risk myelodysplastic syndromes who have these complications even without therapy. Infections were the main cause of death on the study drugs and occurred despite the routine use of antibacterial, azole antifungal, and antiviral prophylaxis at our institution. In contrast to experience with chronic lymphocytic leukaemia or acute myeloid leukaemia, no occurrence of tumour lysis syndrome was observed in this small patient cohort given the prophylactic measures used, including allopurinol and oral hydration.
The combination regimen led to an overall response rate of 87%, consisting of 13% complete remission and 74% marrow complete remission. This rate of response was higher than historically reported with azacitidine monotherapy, which yields response rates of roughly 26%, consisting of 17% complete remission and 9% marrow complete remission. The majority of responses with the azacitidine plus venetoclax combination were marrow complete remissions, indicating that this regimen is highly effective in reducing the bone marrow blast burden. However, venetoclax is known to be highly myelosuppressive in myeloid disorders, and ongoing venetoclax administration as part of the combination regimen might have prevented many patients from meeting count recovery criteria for a full complete remission despite adequate disease control. Although the maximum tolerated dose was not reached, a longer duration of venetoclax is unlikely to have provided increased clinical benefit given the already high response rates and risk of worsening toxicity. The overall response rate observed in our study was generally in line with that seen in another phase 1 study of azacitidine plus venetoclax in high-risk myelodysplastic syndromes, in which Garcia and colleagues described an overall response rate in 44 patients representing 77% of 57 patients, including complete remission in 24 patients representing 42% and marrow complete remission in 20 patients representing 35%. The reason for the higher full complete remission rate in this study is unclear but might be related to the exclusion of therapy-related myelodysplastic syndrome cases and patients after hypomethylating agent failure. These high-risk patient groups were included in our study. In addition, HSCT-eligible patients were excluded from Garcia and colleagues’ study but included in our study. These patients might have gone off protocol for HSCT before achieving their best response if they had continued on azacitidine plus venetoclax. Real-world studies have reported overall response rates of 69 to 75% in frontline patients with high-risk myelodysplastic syndrome treated with hypomethylating agent plus venetoclax.
6 patients with chronic myelomonocytic leukaemia were included in this phase 1 trial, as a retrospective study suggested activity of venetoclax-based regimens in chronic myelomonocytic leukaemia with elevated bone marrow blasts. Although not specifically designed for chronic myelomonocytic leukaemia, the IPSS was used to select these cases for the sake of uniform inclusion criteria in this study. The included patients with chronic myelomonocytic leukaemia were also higher risk when stratified using CPSS-Mol. Our results in this small sample are encouraging with 100% overall response rate and a median overall survival of 14.5 months. The phase 2 portion of this study will further explore the activity of venetoclax in patients with chronic myelomonocytic leukaemia.
Responses to the azacitidine plus venetoclax combination were observed across genetic categories of high-risk myelodysplastic syndromes. Notably, responses were obtained in 4 patients representing 67% of 6 patients with TP53 mutations, a group that is characterised by refractoriness to conventional therapies and a very poor prognosis. Gangat and colleagues reported an overall response rate of 60%, representing 9 of 15 patients, with azacitidine plus venetoclax in patients with myelodysplastic syndromes with excess blasts harbouring TP53 mutations, further supporting the use of the combination therapy in this context. Interestingly, ASXL1 mutations in myelodysplastic syndromes have been associated with heightened sensitivity and improved survival when treated with a hypomethylating agent plus venetoclax. We observed an 89% overall response rate, representing 8 of 9 patients, with 4 having myelodysplastic syndromes with excess blasts and 4 having chronic myelomonocytic leukaemia, in ASXL1-mutated cases, but no significant differences in overall survival or progression-free survival. Larger studies are needed to better delineate the prognostic effect of specific molecular patterns in high-risk myelodysplastic syndromes treated with a hypomethylating agent plus venetoclax.
A significant clinical and genetic overlap exists between high-risk myelodysplastic syndromes and acute myeloid leukaemia, and the combination of azacitidine plus venetoclax is highly effective in inducing responses in both. These findings have led to ongoing discussions about the optimal blast percentage to distinguish the two entities, with some authors proposing patients with 10 to 30% blasts be considered as eligible for both myelodysplastic syndrome and acute myeloid leukaemia therapies.
Responses with the venetoclax combination in our study occurred rapidly after a median of 1 cycle, and no responses occurred beyond cycle 2. This also compares favourably with historical azacitidine monotherapy data, in which responses might take up to 6 cycles. The rapid responses seen with venetoclax make the combination regimen attractive for HSCT-eligible patients who require rapid disease control before undergoing HSCT. Indeed, all four patients in our study who underwent HSCT did so rapidly, within 3 cycles or less of therapy, and did well with this approach. They all remained alive and in remission at the time of data cutoff.
Patients with high-risk myelodysplastic syndromes who fail hypomethylating agent therapy have poor outcomes. In our study, all 6 hypomethylating agent failure patients achieved a marrow complete remission with the addition of venetoclax. Although the number of patients was small, the median overall survival in the hypomethylating agent failure cohort was 8.3 months, comparing favourably with expected outcomes in this difficult-to-treat population with a median overall survival of 4.3 to 5.6 months. Consistent with our findings, another study that included 44 patients with high-risk myelodysplastic syndromes who had failed hypomethylating agent therapy showed an overall response rate of 38.6%, consisting of 6.8% complete remission and 31.8% marrow complete remission, with a median overall survival of 12.6 months following the addition of venetoclax to hypomethylating agent. Taken together, these data support add-on therapy with venetoclax in patients failing hypomethylating agent monotherapy.
A recent biological study done in a large cohort of samples with myelodysplastic syndromes totalling 379 samples showed that myelodysplastic syndrome can be divided into distinct differentiation states based on immunophenotypic and molecular characteristics that establish the haematopoietic stem and progenitor cell architecture. Two different myelodysplastic syndrome phenotypic patterns, namely common myeloid progenitor-pattern myelodysplastic syndromes and granulocytic-monocytic progenitor-pattern myelodysplastic syndromes, were identified based on the frequencies of common myeloid progenitors and granulocytic-monocytic progenitors in the progenitor compartment. In both myelodysplastic syndrome types, haematopoietic stem and progenitor cell populations in distinct differentiation states, meaning long-term haematopoietic stem cells in common myeloid progenitor-pattern myelodysplastic syndrome and lymphoid-primed multipotent progenitors in granulocytic-monocytic progenitor-pattern myelodysplastic syndrome, maintained the disease during hypomethylating agent treatment and expanded after hypomethylating agent failure, thereby driving disease progression. The expansion of each of these haematopoietic stem and progenitor cell types at the time of progression depended on the selective activation of survival pathways mediated by BCL-2 in common myeloid progenitor-pattern myelodysplastic syndrome or NF-kappaB in granulocytic-monocytic progenitor-pattern myelodysplastic syndrome. These data suggest that only common myeloid progenitor-pattern myelodysplastic syndrome might benefit from the addition of venetoclax following hypomethylating agent failure and have implications for improved stratification of patients into venetoclax-based clinical trials. Our study included too few patients with previous hypomethylating agent failure to allow for a dedicated analysis of outcomes based on differentiation pattern. However, we hope further patient accrual into the phase 2 portion will allow for such analysis.
Standard-of-care therapy for HSCT-ineligible patients with high-risk myelodysplastic syndromes consists of hypomethylating agent continued indefinitely until unacceptable toxicity or disease progression. In this context, overall survival is the most important outcome measure. In the hypomethylating agent-naive cohort of our study, the median overall survival with venetoclax plus azacitidine has not yet been reached and a similar finding was also observed by Garcia and colleagues. These observations are encouraging although the data are still preliminary and further follow-up is needed. Ultimately, the question of whether the addition of venetoclax to azacitidine improves overall survival in patients with high-risk myelodysplastic syndromes will be answered by the ongoing phase 3, randomised, controlled trial VERONA.